Johnson & Johnson

What is head and neck cancer?
27 Feb 2026
Learn more about head and neck cancer symptoms and Johnson & Johnson's work to innovate and change current treatments.

“My company makes the medication that helped me fight multiple myeloma”
27 Feb 2026
Paul Reidy, a J&J warehouse technician, never imagined that a multiple myeloma therapy made at the facility where he works would help save his life.

Shockwave: Inside the development of a cutting-edge system to treat coronary artery disease
29 Jan 2026
Learn how Johnson and Johnson's innovative intravascular lithotripsy technology is making treatment safer for certain types of cardiovascular disease.

What is asthma?
27 Jan 2026
Learn about the two types of asthma, symptoms of asthma and how Johnson & Johnson's innovation may lead to better treatments for asthma.

Johnson & Johnson named a 2026
26 Jan 2026
The company made the prestigious list for the 24th consecutive year, thanks to its dedication to innovation and social responsibility, among other criteria.

What you need to know about Johnson & Johnson’s fourth-quarter and full-year 2025 earnings report
21 Jan 2026
See an infographic breakdown of the Johnson & Johnson full-year 2025 earnings report.

What are biologics?
12 Jan 2026
Learn more about how Johnson & Johnson is working to innovate in biologic medications to improve treatment options around the world.
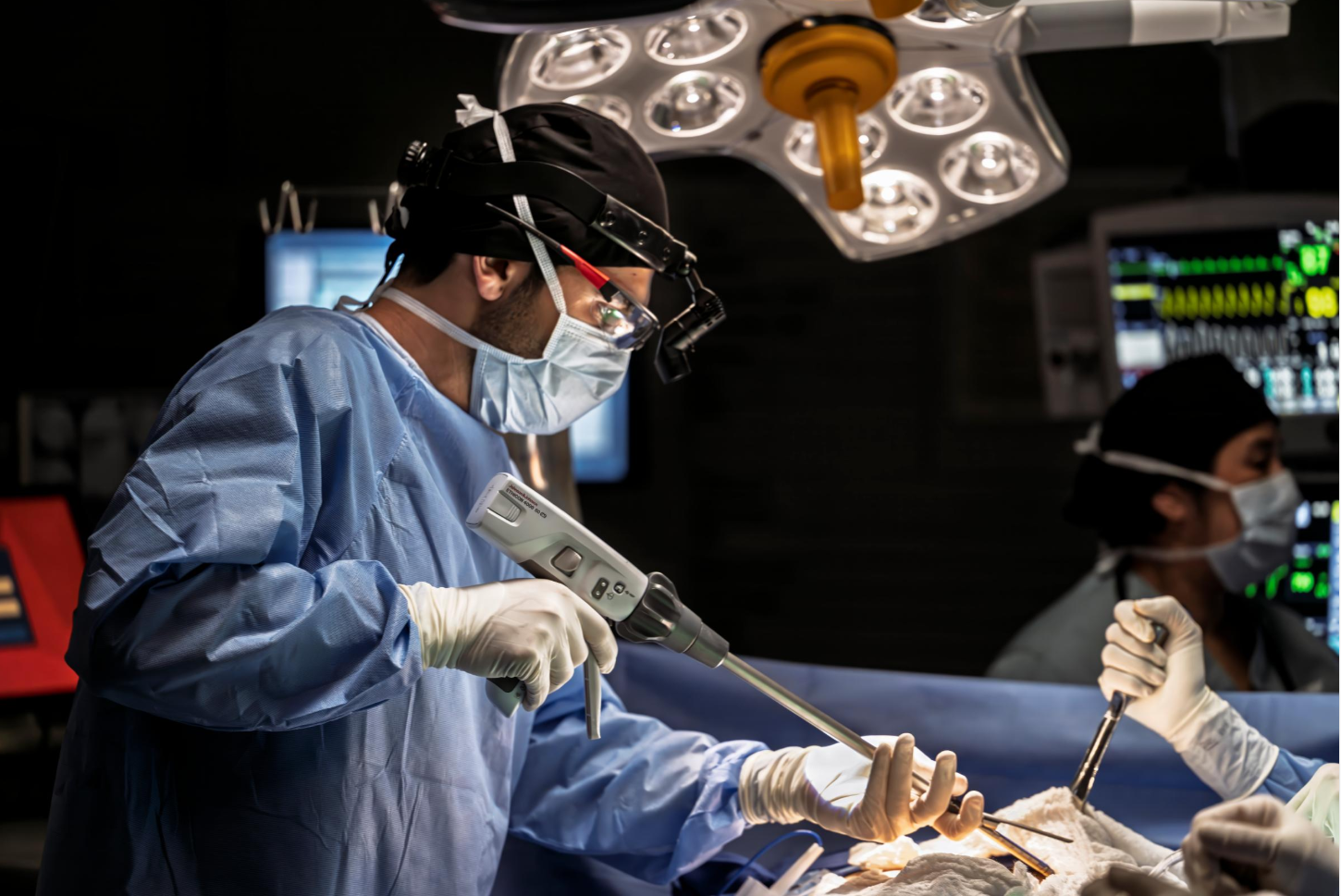
Advances in surgical stapling
12 Jan 2026
Learn about the importance of evolving technology to remove and rejoin tissue in today's changing patient population.

Moving toward a more personalized approach to treating depression
13 Nov 2025
Learn more about how J&J is working to innovate more personalized treatment for major depressive disorder.

“Our goal is a solution for every bladder cancer patient”
10 Nov 2025
Learn how J&J scientist Dr. Christopher Cutie is helping change the treatment landscape for bladder cancer.
Featured

Typewriters, the office machines that preceded computers
By Kiron Kasbekar | 10 Apr 2025
You see office tables today equipped with desktop computers or laptops. But think of the 1970s, and what would you have been seeing?

Wishful thinking about cars
By Kiron Kasbekar | 05 Apr 2025
Donald Trump may be many things, but a good economist he is not. Accustomed to dictating terms to people he has worked with,

The history of safety glass
By Kiron Kasbekar | 26 Mar 2025
You probably already knew that the world’s VIPs move around in cars with bullet-proof glass windscreen and windows. But did you know that ‘bulletproof glass’ is not really bulletproof?

German silver: used in cutlery, music, electricals - but it’s not silver
By Kiron Kasbekar | 20 Mar 2025
This material, which was first developed in China, not Germany, and is an alloy of copper, nickel and zinc, has lost its sheen in home uses, but finds favor in electrical engineering.

Pioneers – the Wrights and Glenn Curtiss launched the aircraft industry
By Kiron Kasbekar | 17 Mar 2025
First Orville and Wilbur Wright flew their plane, the ‘Wright Flyer’, from near a small town called Kitty Hawk. Then Glenn Curtiss built planes with a very different system of controls, which has lasted until now.

What do aircraft have in common with bicycles and motorcycles?
By Kiron Kasbekar | 17 Mar 2025
From chains and sprockets to direct drives—If someone asked you what aircraft have in common with bicycles and motorcycles, how would you respond?

Radar’s ancestors: From sound mirrors to modern detection technology
By Kiron Kasbekar | 11 Mar 2025
Radar has become a well-settled technology today, especially in the field of navigation.

Nokia Bell Labs: innovations in communication, computing, technology
By Omar Almeida | 08 Mar 2025
Bell Laboratories, or Bell labs, which has now become Nokia Bell Labs, is one of the most renowned research and development organizations in the history of science and technology.

Company story – Quaker Oats
By Kiron Kasbekar | 08 Mar 2024
Quaker Oats is a company whose products I remember from my childhood days. Years before a foreign exchange crisis caused the Indian government to impose curbs on consumer product imports, we used to see a host of foreign brands in the Indian market. Including Quaker Oats, which I remember eating when I was a child, and which has been available for the past two decades or more.









